find the number of electrons co|How to Find Number of Protons, Neutrons, and Electrons : Pilipinas Find the element Co Co on the periodic table. . To find the number of electrons in cobalt cobalt, first locate the element on the periodic table. Next, find the atomic number which is located above the element 's symbol. Since cobalt cobalt 's atomic number is 27 27, Co Co has 27 27 electrons. Confira o resultado da Nacional Loterias de hoje, sorteio da L.
find the number of electrons co,Find the element Co Co on the periodic table. . To find the number of electrons in cobalt cobalt, first locate the element on the periodic table. Next, find the atomic number which is located above the element 's symbol. Since cobalt cobalt 's atomic number is 27 27, Co Co has 27 27 electrons.
In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Cobalt (Co). From the Periodic.

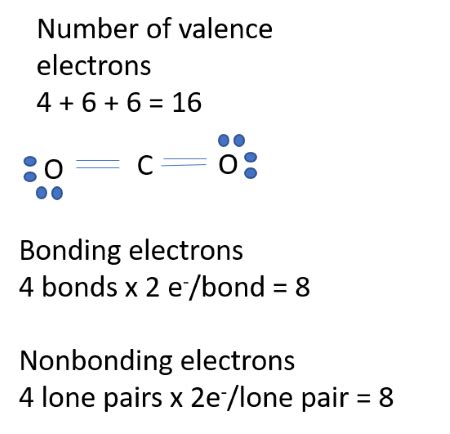
The total number of electrons in CO 2 areA. 22B. 44C. 66D. 88 - BYJU'S
How To Calculate The Number of Protons, Neutrons, and ElectronsHow to Find Number of Protons, Neutrons, and ElectronsThe total number of electrons in one molecule of carbon dioxide is - To.The total number of electrons in CO 2 areA. 22B. 44C. 66D. 88 - BYJU'S
The correct option is A 22. Number of electrons in a molecule is equal to the sum of electrons in individual atoms in it. And electrons in an atom is equal to its atomic number. In CO2, there . Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons in an atom. Write and interpret symbols that .
Question. Calculate the number of electrons in 100 grams of CO2. Can a body have a charge of (a) 0.32×10−18C (b) 0.64×10−20C (c) 4.8×10−21C? Solution. Verified by Toppr. No of moles of .
Solution. Verified by Toppr. The atomic numbers of carbon and oxygen are 6 and 8 respectively. Thus, they contain 6 and 8 electrons respectively. The number of total electrons present in one .
The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom components - protons, neutrons, and electrons (or vice .
The easiest way to find the atomic number, is to look on a periodic table, the atomic number is in the upper left corner, or is the largest number on the square. Finding the Number of Protons. .How to calculate the number of protons, neutrons, and electrons using the atomic number, mass number and some key correlations.
How to easily find the number of electrons, protons and neutrons in a cobalt atom? . Co – 3e – → Co 3+ Here, the electron configuration of cobalt ion(Co 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. This cobalt ion(Co 3+) has twenty-seven protons, thirty-two neutrons, and twenty-four electrons.The unpaired electrons in $$[Ni(CO)_4]$$ are: A. zero. B. four. C. three. D. one. Open in App. Solution. Verified by Toppr. Correct option is A. zero. Was this answer helpful? 6. Similar Questions. Q1. . Out of this, select the complex with m aximum number of unpaired electrons. Look for the atomic mass of the element. To find the number of neutrons, you will first need to find the atomic mass. An element’s atomic mass (also known as the atomic weight) is the weighted average mass of atoms of .

Identify the charge of the ion. Adding and removing electrons from an atom does not change its identity, but it changes its charge. In these cases, you now have an ion, such as K +, Ca 2+, or N 3-.Usually, the charge is expressed in .
The magnetic moment is related to the number of unpaired electrons according to the following relation: Magnetic moment, μ = √ n ( n + 2 ) B M where, n = number of unpaired electrons. B M stands for Bohr magneton, a unit of the magnetic moment.The number of unpaired electrons in Co in the complex ion [C o (C 2 O 4) 3] 3 − is [Atomic number of C o = 27] Q. Write the hybridisation and number of unpaired electrons in the complex [ C o F 6 ] 3 − .find the number of electrons coStep 4: Identify the number of electrons unaccounted for. We have thus far determined the single bond connectivity for (CH), CO as: Draw the Lewis structure of acetone, (CH),CO. Step 1: Identify the number of valence electrons. Step 2: Identify the central atom. Step 3: Establish connectivity of single bonds.Q. Calculate the total number of electrons present in 1.6 gm of methane. Q. Calculate the number of electrons in 16 gm O 2 − . Q. Molecular nitrogen can be converted into NO by the following reaction sequence, N 2 + 3 H 2 → 2 N H 3The number of unpaired electrons in Co in the complex ion [C o (C 2 O 4) 3] 3 .The anion [N i C l 4] 2 − is paramagnetic but when C N − ions are added, the product [N i (C N) 4] 2 − is diamagnetic because in [N i C l 4] 2 −, C l − is a weak ligand and thus, electrons are unpaired in N i 2 + making it paramagnetic, s p 3 hybridised and tetrahedral.
Determine the number of unpaired electrons, magnetic moment, and ligand field stabilization energy for each of the following complexes: a. [Co(CO)4)- b. [Cr(CN).]Find the maximum number of electron that are involved in shielding of an electron of Ni atom having quantum numbers. n = 2, l = 1 ,m = 0 , s = +1/2 .
How many unpaired electrons are present in $$ Co^{3 +} $$ ion? View Solution. Q5. How many atoms are represented in N a 2 C O 3 .Calculate the total number of unpaired electrons in [Co(H 2 O) 6] 2 +. Open in App. Solution. Verified by Toppr [C o (H 2 O) 6] 2 + has three unpaired electrons. It has 3 d 7 outer electronic configuration which can also be written as t 5 2 g e 2 g. . Find the number of unpaired electrons calculated in [Co .
The number of unpaired electrons in the complex ion [C o F 6] 3 − is (atomic no. C o = 27): View Solution. Q4. The number of unpaired electrons in the complex ion [C o F 6] 3 − is (Atomic number Co=27) View Solution. Q5. The number of unpaired electrons in the complex ion .
M n 3 +: 3 d 4, Number of unpaired electrons is 4. C r 3 +: 3 d 3, Number of unpaired electrons is 3. V 3 +: 3 d 2, Number of unpaired electrons is 2. T i 3 +: 3 d 4, Number of unpaired electrons is 1. C r 3 + is most stable in aqueous solution.
Number of electrons present in hydrogen molecule (H 2) = 1 + 1 = 2. ∴ Number of electrons in = 2 – 1 = 1. H 2: Number of electrons in H 2 = 1 + 1 = 2: Number of electrons present in oxygen molecule (O 2) = 8 + 8 = 16. ∴ Number of electrons in = 16 – 1 = 15Co 2 + (d 7) in [I I C O (SCN) 4] 2 − has outer electronic configuration 3 d 7 or e 4 g t 3 2 g. e g orbitals have lobes present along the axis. They contain 0 unpaired electrons. Thus, the number of unpaired electron present in the d-orbitals (whose lobes are present along the axis) for the complex [CO(SCN) 4] 2 − is 0.
find the number of electrons co|How to Find Number of Protons, Neutrons, and Electrons
PH0 · The total number of electrons in one molecule of carbon dioxide
PH1 · The total number of electrons in CO 2 areA. 22B. 44C. 66D. 88
PH2 · How to find the Number of Protons, Electrons, Neutrons for Co
PH3 · How to Find Number of Protons, Neutrons, and Electrons
PH4 · How to Find Electrons: 6 Steps (with Pictures)
PH5 · How To Calculate The Number of Protons, Neutrons,
PH6 · Find the Number of Electrons Co
PH7 · Calculate the number of electrons in 100 grams of {CO}
PH8 · Atom Calculator
PH9 · 2.6: Protons, Neutrons, and Electrons in Atoms